Solar energy
The purpose of this page is to give some of the basic physics about where the energy comes from that is used in renewable energy systems. Rather than starting at the usual place, down here on Earth, this will take us further back to the ultimate source, the sun. Attempts will be made to keep the explanations as simple as possible while still offering a lower level of understanding.
Atoms, protons, electrons
All matter is made up of atoms. Your body, for example, is made up of countless of these very, very small atoms. Using a simple model of the atom, an atom consists of a nucleus around which electrons rotate. The nucleus is made up of neutrons and protons.
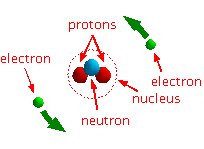
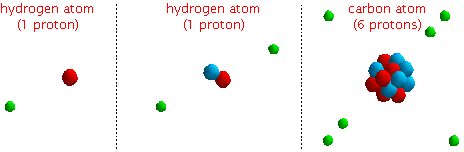
Different types of atoms have different numbers of protons in the nucleus. That's how we distinguish between the different types.

|
Two hydrogen atoms are in a bar. One turns to the other and says "I think
I've lost an electron." |
|
A neutron walks into a bar and asks "How much for a drink?" |

|
What is energy?
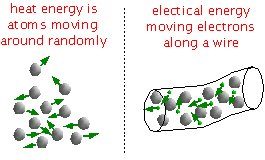
Put simply, energy is defined as the potential for moving things around. In physics this is considered doing work. There are many forms of energy. For example, heat energy is atoms moving around randomly. The more movement there is the hotter we say it is. Electrical energy moves electrons along a wire (electrons don't always stay with their atoms).
Nuclear fusion... or how the sun provides energy
The energy we use from the sun arrives in the form of sunlight, a form of electromagnetic energy. We convert this to heat energy in a solar hot water heater to heat water and we convert it to electrical energy in a solar power, or photovoltaic, panel to produce electricity. But how does the sun produce that sunlight?
When we described what makes up an atom, we said the nucleus is made up of neutrons and protons. It takes energy to hold these neutrons and protons together into a nucleus (there are even smaller particles inside the neutrons and protons that do this work.) This energy comes in the form of a force is called the nuclear force, which makes sense as it is the force holding the nucleus together.
The interesting thing (see the diagram below) is that it takes less energy to hold a neutron and two protons together (an helium atom) than the total energy holding together a hydrogen atom consisting of just a proton and another hydrogen atom consisting of a proton and a neutron.
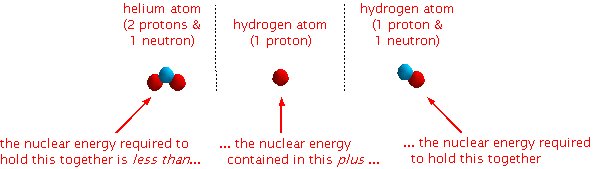
So if you combine the two hydrogen atoms you get a helium atom plus some leftover energy. In this particular example, the leftover energy will be in the form of a very high energy photon.
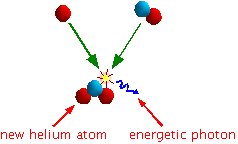
Another word we could have used instead of "combine" is "fuse". We have fused the two hydrogen together, resulting in helium. We have done nuclear fusion. This is an example of the type of fusion going on inside our sun. There is also nuclear fusion going on involving other, larger atoms resulting in even more energy being released.
But didn't it take energy to pull the two hydrogen atoms together? The answer is yes, and in the case of the sun, this energy is provided by gravity. Wherever you have matter, then you have gravity. The more matter you have, the stronger the gravity. The sun has an enormous amount of matter and so the resulting gravity is also enormous. It is this gravitational energy that pulls the atoms together. But that alone doesn't fuse them.
There's a force called the Coulomb force that prevents them from fusing, or coming together.
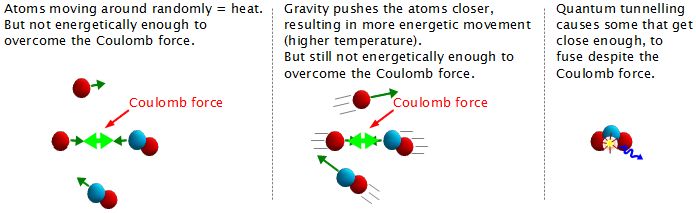
As we said above, heat energy is atoms moving around randomly. The more energetically they move around, the hotter it is. The enormous gravity in the sun compresses the atoms into a smaller volume and they therefore move around very energetically; the temperature is very high. The result is that these energetically moving atoms end up getting very close together at times. But in our sun they aren't moving energetically enough to overcome the Coulomb force and fuse; i.e. the temperature isn't hot enough.
The final ingredient is quantum tunneling. Quantum tunneling is a process whereby if the atoms get close enough together, even if they don't come close enough to fuse on their own, some of them will fuse anyway. They sort of tunnel through the barrier made by the Coulomb force. And as we said above, one of the results of the fusion is the release of a high energy photon.
This photon doesn't actually leave the sun. Instead it participates in other fusion events, or it transfers its energy to other atoms making them move more energetically, i.e. transforms into heat energy. Eventually some of this energy makes its way up through the layers of the sun until it finally escapes from the sun in the form of very fast moving photons.
Photons can be thought of as either particles or waves. As you can see from the diagrams, we're using a combination of these to represent a photon, a wavey particle.
It's these energetic photons that arrive at the Earth and are converted by solar panels to electrical energy, by solar water and solar air heaters to heat energy, by heating large volumes of air to wind energy, and so on. All forms of energy on Earth came from the stars, of which our sun is one, although you may have to trace through a few steps of energy conversion to realize it.
The conservation of energy law
The total amount of energy in the universe never changes. Energy can neither be created nor destroyed (this is the conservation of energy law which you may have heard of.) But if that's the case, why can't we just place our solar power panels in the sun, let the panels convert the energy from the photons into electrical energy to move electrons along wires, and then put the solar panels away? After all, energy is never destroyed. That's true but energy can change from one form to another.
As the electrons move down a wire they cause some of the atoms to start moving around, as if the electrons were "bumping" into them. Two things happen because of this bumping. The moving atoms cause heat since, remember, that's what heat means, randomly moving atoms. And the electrons move a little less, having lost a little energy through bumping. So the amount of heat energy has increased while the amount of electrical energy has decreased by the exact same amount. The total amount of energy hasn't changed. So to make up for the energy that was converted to heat energy, we need to keep converting the more energy from photons to more electrical energy.
